Chapter 1. Overview of the Canadian Task Force on Preventive Health Care
Last updated: January 27, 2022
1 Overview of the Canadian Task Force on Preventive Health Care
1.4 Canadian Task Force on Preventive Health Care Membership
1.5.1 Role of the Canadian Task Force on Preventive Health Care
1.5.3 Roles of External Partners
1.6.1 Guideline Working Groups
1 Overview of the Canadian Task Force on Preventive Health Care
1.1 Mandate
The mandate of the Canadian Task Force on Preventive Health Care is to develop and disseminate evidence-based recommendations to support primary care practitioners in their delivery of preventive health care for Canadians.
The Task Force uses the following definition of primary care based on the World Health Organization, Europe (1):
Primary care is a key process in the health system. It is first-contact, accessible, ongoing, comprehensive and coordinated care. First-contact care is accessible at the time of need; continued care focuses on the long-term health of a person as well as the short duration of the disease; comprehensive care is a range of services appropriate to the common problems in the respective population, and; coordination is the role by which primary care acts to coordinate other specialists that the patient may need.
The recommendations of the Task Force are aimed at improving clinical practice relevant to primary or secondary disease prevention. Primary prevention is directed at preventing a medical condition or injury in healthy patients before it occurs, while secondary prevention is designed to reduce the impact of disease or injury that is already present but may not be clinically evident (2).
1.2 Structure and Function
The Task Force is an independent panel, composed of clinicians and research and guideline methodologists. The Task Force makes recommendations on preventive health care using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (3) based on rigorous evidence syntheses. The GRADE approach provides a structured and transparent process for rating the certainty of evidence, weighing benefits and harms, grading strength of recommendations, as well as considering other important factors such as patient values and preferences, resource use, equity, acceptability and feasibility in making recommendations (3).
The Task Force develops guideline recommendations for Canadian primary care providers, policy makers, and patients, its key knowledge users. Its work is also relevant to other health care and public health providers, program developers and Canadian citizens seeking evidence-based recommendations on preventive health care. The Task Force develops and fosters linkages between primary care, community and public health programs that support preventive health care interventions, as well as linkages to enhance the dissemination and uptake of its recommendations. Finally, the Task Force works with researchers to advance the evidence base supporting preventive care interventions and the methodology for guideline development.
The Task Force contributes to improvements in the quality of health care by:
- Producing high quality, evidence-based guidelines for preventive health care interventions, primarily for use by primary care providers, policy makers, and patients.
- Focusing on primary care health questions and interventions that will have the greatest impact in improving the health of Canadians.
- Selecting and evaluating prevention interventions for clinically relevant conditions.
- Working with ERSCs to develop criteria for the selection of evidence and interpret findings from evidence syntheses.
- Identifying gaps in current knowledge in clinical prevention interventions.
- Engaging specialists and experts from other disciplines to ensure that disease-specific knowledge is integrated with Task Force expertise on screening and prevention.
- Engaging patients and members of the public to incorporate their values and preferences in development of guidelines and decision-making tools for clinical practice.
- Working with the KT Team to develop and disseminate tools and materials to help primary health care providers apply recommendations in practice.
- Engaging key stakeholders in guideline development and topic selection to ensure relevancy of guidelines to a wide audience and high quality of guidelines via stakeholders’ expert input.
1.3 Governance
The Task Force has independent decision-making authority in all aspects of its mission, including the following activities:
- Obtaining recommendations from stakeholders and the public
- Choosing topics and guideline research questions
- Composition of guideline development teams
- Selection of ERSC teams used to undertake evidence reviews
- Setting of methods and expectations for review and synthesis of the evidence
- Development, public declaration, and dissemination of its recommendations
- Organizing Task Force and external stakeholder meetings
- Mentoring Task Force fellows
- Participating in guideline-related outreach activities
- Media and social media interactions
1.4 Canadian Task Force on Preventive Health Care Membership
The Chair, Vice-Chair and members (no more than fifteen, including the Chair and Vice-Chair) are jointly appointed by the Chief Public Health Officer of Canada (CPHO) and the Executive Director, Professional Development and Practice Support of the College of Family Physicians of Canada (CFPC).
The Task Force will periodically solicit applications for new members, including the Chair and Vice-Chair, by contacting appropriate stakeholder groups and through other appropriate channels. PHAC does not propose individuals for consideration as future members.
Selection and Appointment of New Members:
Once one or more individuals have applied for membership, the Task Force will appoint a Selection Committee composed of the Task Force Chair and Vice-Chair, two Task Force members, one PHAC representative, and the Executive Director, Professional Development and Practice Support (or a delegate) of the CFPC. The Chair and Vice-Chair will be co-chairs of the Selection Committee. The Selection Committee will review the qualifications of each applicant in relation to the required qualifications for selection (see Table 1 below) and will make its recommendations for appointments to the Task Force. The Task Force will vote to approve or not to approve the recommendations. After the Selection Committee and Task Force membership have reached a decision, the candidate is notified.
PHAC and CFPC will appoint new members after receipt of the recommendation from the Selection Committee and confirmation by consensus from the Task Force membership. The CPHO and the Executive Director, Professional Development and Practice Support of the CFPC will execute appointment of new members after receipt of the recommendations from the Task Force by signing appointment letters.
All applications will be reviewed by the full Selection Committee unless pre-reviewed by a sub-committee, formed by the Chair, the Vice-Chair, and PHAC representative, that deems an application would not stand a chance for membership recommendation by the full committee. To have a candidate removed from consideration, the sub-committee needs to unanimously agree that the candidate would not be given serious consideration for membership by the full committee.
Table 1. Qualifications for appointment to the Task Force
- Expertise in disease prevention and health promotion of children, youth and/or adults
- Experience in systematic reviews, their methodology and their application to clinical decision-making and/or policy
- Experience in guideline production and dissemination
- Ability to be rigorous but open-minded when reviewing and interpreting scientific evidence and associated recommendations
- Knowledge and experience in critical appraisal of peer-reviewed publications
- Ability to work collaboratively with peers
- Ability to allocate the necessary time to participate actively in the Task Force
- Working in Canadian health care or university setting
- Strongest consideration will be given to individuals who are widely recognized for scholarly leadership within their fields of expertise
- A background in primary care practice will be considered an asset
- No significant secondary interests (including financial or intellectual conflicting interests) that would impair the integrity of the Task Force
Adapted from the procedure manual of the US Preventive Services Task Force (4).
Selection and Appointment of the Chair and Vice-Chair:
Following the receipt of applications, PHAC will strike a Selection Committee to review the qualifications (see Table 2 below) of each applicant for the Task Force Chair and Vice-Chair positions. The Committee will be co-chaired by the Director General of the Centre for Chronic Disease Prevention and Health Equity at PHAC and the Executive Director, Professional Development and Practice Support of the CFPC Other members will include the current Task Force Chair and Vice-Chair, one current and one past/outgoing Task Force member, as well as an additional member of the CFPC, and a Canadian Medical Association member, both of whom must have experience in research methods and guideline development. If the Vice-Chair is applying for the Chair position, an additional Task Force member will be added to the committee in the Vice-Chair’s place. Based on recommendations of the Committee, the committee co-chairs will propose nominees for Task Force Chair and a Vice-Chair for appointment by the CPHO and CFPC.
Table 2. Qualifications for Chair/Vice-Chair
In addition to the qualifications listed in Table 1,
- Excellent skills in consensus building, negotiating, and conflict resolution
- Comfortable speaking on behalf of the Task Force in public venues
- Evidence of having participated in (preferably led) end-to-end development of evidence-based guidelines
- Evidence of leadership experience with a similarly sized group or entity, from organizational, scientific, interpersonal, financial/budget perspectives
- Performance on the Task Force
- Strongest consideration will be given to individuals who:
- Are well networked within the healthcare sector and public health and are recognized nationally or internationally for scientific leadership within their field of expertise.
- Have presence and excellent interpersonal and communication skills to champion the rigour behind the methodology and scientific approach.
- Have proven their ability to encourage consensus and collaboration among members of working groups, committees or boards.
- Have successfully facilitated and developed evidence-based guidelines and recommendations.
Note: The Chair, Vice-Chair or both should be a family physician and member of the CFPC.
1.4.1 Terms of Service
The Chair, Vice-Chair and members will be appointed for a term of four years, renewable once.
Terms of less than four years for Chair and Vice-Chair may be considered on an exceptional basis, at the discretion of the Selection Committee.
Members of the Task Force who have previously served two terms on the Task Force should be restricted to one term as Chair.
A member may be extended for a time limited period to complete work on specific guidelines at the discretion of PHAC and CFPC.
Appointments will be staggered to ensure continuity of membership over time and overlap of terms including the Chair and Vice-Chair.
1.5 Overview of Roles
1.5.1 Role of the Canadian Task Force on Preventive Health Care
The Task Force is primarily responsible for prioritizing and selecting topics, developing research questions, informing appropriate evidence review methodologies and providing pragmatic primary care clinical input that aligns with the health needs of Canadians, and considering external stakeholder input at various steps across the guideline development process. The Task Force is responsible for developing, publishing and disseminating recommendations for each topic they select based on review of the best available evidence as well as ongoing evidence surveillance to support guideline updates. Task Force members are encouraged to discuss, disseminate, and support the recommendations of the Task Force in public forums and social media (e.g. Twitter, Instagram).
1.5.1.1 Role of the Task Force Office
The Task Force Office is based at the institution of the Task Force Chair and is established via a Contribution Agreement between PHAC and the named institution. Under the agreement, the named institution provides administrative support for the Task Force, stakeholder engagement, evaluation, and funding for knowledge translation, communication and ERSCs.
1.5.1.2 Role of Task Force Fellows
The Task Force is committed to providing mentored training opportunities to Canadian healthcare trainees and early career professionals. Opportunities for Task Force fellows include short-term training opportunities and longer-term fellowships. Both short-term and longer-term fellowships involve working closely with a Task Force member who will supervise and provide mentorship to the fellow.
Task Force fellows do not vote on the direction or strength of recommendations. Exceptionally, longer-term fellows who engage with a Task Force Working Group Chair from the initiation to the completion of a guideline may be eligible to be considered for authorship. If they are included as a guideline author, it will be clearly noted in the guideline publication that they participated as a “non-voting Task Force fellow” and not a member of the Task Force.
1.5.2 Roles of Core Partners
1.5.2.1 Role of the Science Team from the Public Health Agency of Canada
The Science Team at PHAC provides guideline development expertise and organizational support to the Task Force. One or more Science Team members are assigned for each guideline topic to coordinate and provide scientific support at each step of the process, including scoping a new guideline topic and writing the first draft of the guideline manuscript. The Science Team offers a secretariat function for both guideline and functional (e.g., Methods, Topics) working groups of the Task Force and is responsible for managing logistics of working groups and guideline timelines.
As noted above, the Task Force maintains independent decision-making authority and as such, while PHAC provides epidemiological and project management support via the Science Team, the Science Team does not have any input on the topics selected by the Task Force or their recommendations.
1.5.2.2 Role of the Evidence Review and Synthesis Centres
The ERSCs are contracted to conduct and/or assess the evidence syntheses (e.g., systematic reviews) used as the evidence base for the Task Force’s recommendations. The ERSCs follow standard methods for their reviews of the topics specified by the Task Force based on Cochrane methods (5), the GRADE approach (3) and this methods manual (see Chapter 4). In the event where these sources conflict, this methods manual will take precedence. The ERSC leads the writing of the protocols and evidence reviews. The ERSCs also provide scientific support on various Task Force functional working groups (e.g., Methods, Knowledge Translation).
1.5.2.3 Role of the Knowledge Translation Team
The KT Team develops tailored messages and tools for clinicians and patients, aids in guideline implementation and evaluation projects, and is responsible for review and incorporation of emerging best practices for knowledge translation.
The KT Team also executes the work approved by the KT Working Group and engages with the Communications Team for matters related to public relations.
1.5.2.4 Role of the Communications Team
The Communications Team works closely with the KT Team, Task Force Chair, Vice-Chair, guideline working groups and Science Team to set communications direction, provide advice, create strategies, messaging, materials, and tactics to communicate Task Force content and guidelines to stakeholders and the public. This includes monitoring and managing social media channels as well as traditional media sources. Reputation and issues management is also a key function.
The team also provides input, when appropriate, into stakeholder materials created by other teams and Task Force members.
1.5.2.5 Role of Task Force Public Advisors Network
The Task Force Public Advisors Network (TF-PAN) is an initiative being piloted by the Task Force. The TF-PAN is comprised of members of the public who provide meaningful input throughout the development and dissemination of Task Force guidelines. More detail on TF-PAN methods will be added to this manual following the pilot phase.
1.5.3 Roles of External Partners
1.5.3.1 Role of Clinical and Content Experts
Clinical and content experts are specialists or other topic experts invited to serve as advisors to guideline working groups, providing valuable insight and expertise throughout the guideline development process. These experts are engaged early in and throughout the guideline development process. They provide the working group with insight on complex clinical matters, review documents for accuracy and participate in meetings, as needed. Clinical and content experts do not have input into, or vote on, the direction or strength of recommendations.
1.5.3.2 Role of Stakeholders and Peer Reviewers
External linkages are developed with health care organizations (e.g., professional associations), government-related organizations, patient/public organizations and, in certain cases, selected groups of practitioners. Such organizations represent those who use Task Force recommendations in their day-to-day practice. External linkages may also be set up with organizations that are in a position to enhance uptake of Task Force recommendations.
Through such organizational linkages, the Task Force identifies stakeholder groups and peer reviewers whose main function is to inform guideline development and provide effective and meaningful input throughout the process as further described in Chapter 6. External stakeholders and peer reviewers serve a variety of functions, including:
- Suggestions of topics that might be considered for a guideline
- Review of protocols, evidence reviews, and draft recommendations
- Consultation on guideline implementation and guideline dissemination
- User testing of KT tools
- Endorsement of guidelines
- Facilitate Task Force presentations to stakeholder groups
1.6 Working Groups
1.6.1 Guideline Working Groups
Each guideline working group consists of a minimum of three and a maximum of six members of the Task Force (larger working groups may be established in exceptional cases). Working group members direct each step of the guideline development process and ultimately develop the recommendations which are voted on by all members of the Task Force. Each guideline working group is supported by a Science Team Lead from PHAC, members from the ERSCs and clinical/content experts. It is important to note that only members of the Task Force generate, discuss, and vote on recommendations.
Members of each guideline working group are expected to actively participate in all aspects of the guideline development process, including developing the questions and analytic framework, reviewing the evidence, and providing input on recommendations. This work involves attending regular conference calls and providing prompt feedback as required.
Each guideline working group will be assigned a Working Group Chair and Vice-Chair. The Task Force Chair and Vice-Chair work with Task Force members to determine working group membership and select the Working Group Chair and Vice-Chair.
The Chair of the working group has the following responsibilities:
- Work with the Science Team Lead to set the agenda, prepare, and chair meetings of the working group,
- Work with Science Team Lead to help ensure work proceeds according to specified timelines,
- Liaise with the ERSCs and Science Team Lead to provide updates about the work and to coordinate meetings,
- Ensure that the scope of the review is clear for all working group members (e.g., the analytic model, key questions, chosen evidence base, and outcomes),
- Ensure that working group members understand the guideline development and evidence-to-decision process in which they are engaged,
- Facilitate working group discussions, encouraging members to share feedback and voice concerns,
- Address issues as they arise and work toward consensus of the working group on guideline decisions.
The Vice-Chair of the working group has the following responsibilities:
- Supporting the Working Group Chair in their role (e.g., providing input on documents and issues to be discussed with working group),
- Fulfilling responsibilities of the Working Group Chair when Chair is unavailable, including chairing of working group meetings and approving documents as needed.
Meetings
- The Science Team schedules meetings for the guideline working group, taking into consideration the Chair’s schedule. The Chair should respond to requests about these meetings from the Science Team or members of the working group.
- Working group members who cannot attend a meeting may provide comments, before or after the meeting, to the Chair, the Science Team Lead, or all working group members.
1.6.2 Functional Working Groups
1.6.2.1 Topics Working Group
The Topics Working Group assists the Task Force to select topics to consider for guideline development, according to the process outlined in Chapter 2 of this manual. Criteria for topic selection have been developed and are applied to ensure transparency, reproducibility, and objectivity in the topic-selection process. On the basis of these criteria, the Topics Working Group solicits and considers input from the Task Force and its partners (core and external) concerning the topics that should be addressed. Topic priorities are re-examined annually.
The Topics Working Group is led by two members of the Task Force (the Working Group Chair and Vice-Chair), composed of other interested individuals from the Task Force, and supported by staff from the Science Team.
1.6.2.2 Methods Working Group
The Methods Working Group assists the Task Force to maintain the highest methodological standards in guideline development. Output from this working group ensures that Task Force guidance and the methods used to produce such guidance are methodologically sound, scientifically defensible, reproducible, and well documented.
The Methods Working Group is responsible for the ongoing review and updating of the Task Force methods manual which documents the methods used by the Task Force, the ERSCs, the KT Team, and the Science Team to develop reviews and recommendations. The Methods Working Group identifies areas where modifications, expansions, or updates are required.
In addition, the Methods Working Group addresses important scientific and methodological issues as they arise, including but not limited to reviewing existing tools for appraisal, making recommendations about the types of studies to be included in evidence reviews, integrating advances to guideline methods and reviewing knowledge translation issues related to guideline development. All decisions related to methods issues are documented in the methods manual.
The Methods Working Group is led by two members of the Task Force (the Working Group Chair and Vice-Chair), composed of other interested individuals from the Task Force, and supported by staff from the Science Team, ERSCs and KT Team.
The process for integrating new methods and making revisions to existing methods is as follows:
- All proposed new methods are first taken to the Methods Working Group for review of background, rationale, and approval to incorporate into Task Force methods.
- Methods may be piloted before a final decision is made to integrate into the standard Task Force methods.
- Once approved by the Methods Working Group, the new methods have to be reviewed and approved by the full Task Force.
- Once approved by the full Task Force, changes are incorporated into the methods manual.
1.6.2.3 Knowledge Translation Working Group
The KT Working Group oversees the development of clinician and patient tools for each guideline. The KT Working Group also develops partnerships and strategies aimed at advancing and supporting the dissemination and uptake of Task Force guidelines and other knowledge products into clinical primary care practice.
The KT Working Group has the following key objectives:
- To disseminate Task Force guidelines to stakeholders through the development of decision tools, publications, presentations, media and communications
- To develop and maintain relationships with a range of stakeholders, including national disease-specific and general organizations, primary care providers, patients and members of the general public, and policy-makers
- To evaluate the impact (dissemination and uptake) of the Task Force guidelines upon primary care providers to ensure that KT activities are effective, appropriate, and consistent
The KT Working Group is led by the KT Team, composed of interested members from the Task Force and supported by staff from the Science Team.
1.6.2.4 Equity Working Group
The Task Force recognizes the importance of advancing health equity and is committed to focusing efforts in this field. The Equity Working Group aims to promote health equity through activities such as determining the current best practices for addressing health equity in guideline development, and developing plans for incorporating health equity in Task Force work.
The Equity Working Group is led by two members of the Task Force (the Working Group Chair and Vice-Chair), composed of other interested individuals from the Task Force, and supported by staff from the Science Team, ERSCs and KT Team.
1.7 Quorum and Voting
A quorum (the minimum number of members present for decisions to be considered valid) for official votes is two-thirds of the members of the Task Force, including the Chairs.
Major decisions about procedures and methods, and the selection of new members, all begin with a non-secret vote. Votes are taken by hand, by voice, or by proxy, and voting can be done electronically if necessary (e.g., if a quorum is not available during an in-person meeting). Votes are recorded as yes, no, or absent.
The Chair and Vice-Chair work towards consensus on decisions by the Task Force. In the case that an insufficient number of Task Force members are present at a meeting for decision-making by consensus (i.e., less than 2/3), those present may still discuss the issue and develop a proposed decision to bring forward (in a meeting or electronically) to those who were not present for the original discussion, to get their consensus.
Consensus means agreement by at least two-thirds of the members (irrespective of presence or absence in decision-making meeting), reached through a process that accounts for the views and concerns of all members.
When consensus cannot be reached, this will be recorded in meeting minutes, and the issue being voted upon will be reconsidered to address concerns raised, so that it can be brought to a subsequent meeting. If needed, the issue will brought to a blinded vote (e.g., using anonymized voting software).
Note that some major Task Force documents require full approval by working group and Task Force members (see section 1.7.2).
1.7.1 Quorum for Working Group Decisions
A quorum for working group decisions (e.g., decisions regarding aspects of guideline scope) is two-thirds of working group members (round to nearest whole number; i.e., 3 of 4, 3 of 5, or 4 of 6 members). Agreement by at least two-thirds of working group members is required for all working group decisions.
1.7.2 Approval of Major Task Force Documents
Products developed throughout the guideline process have different requirements for approval by the guideline working group and full Task Force. Major approval steps are outlined in Table 3.
Table 3. Approval requirements for major Task Force documents
| Guideline Development Stepa | Guideline Working Group Approval | Task Force Approval |
| Clinical expert listb | Working group given opportunity to comment Approval by Working Group Chair only | N/A |
| Stakeholder and peer reviewer listc | Approval by non-objectiond | Approval by Task Force Chair only |
| Key questions and scope (e.g., population, intervention(s), comparator(s), outcomes) | Approval by consensuse | Presented to Task Force for input (Task Force will later approve key questions and scope as part of protocol review) |
| Final scoping document | Working group given opportunity to comment Approval by Working Group Chair only | N/A |
| Final feasibility report | Working group given opportunity to comment Approval by Working Group Chair only | N/A |
| Protocol: pre-journal submission | Full approvalf | Opportunity to comment; approval by non-objection |
| Protocol: journal re-submission (after stakeholder and peer review revisions) | Full approval | Opportunity to comment; approval by non-objection |
| Evidence review manuscript: pre-journal submission | Full approval | Opportunity to comment; approval by non-objection |
| Evidence review manuscript: journal re-submission (after stakeholder and peer review revisions) | Full approval | Opportunity to comment; approval by non-objection |
| Evidence to Decision table (preliminary) | Working group develops and reviews table Approval by Working Group Chair only | Opportunity to comment (Task Force will later approve Evidence to Decision table as part of guideline review) |
| Recommendations | Approval by consensus | Approval by consensus |
| Guideline: pre-external stakeholder review | Full approval | Full approval |
| Guideline: pre-journal submission | Full approval | Full approval |
| Guideline: re-submission (after external review revisions) | Full approval | Full approval |
aTwo rounds of review (minimum) for both working group and Task Force members at each step for protocols, evidence reviews and guidelines. If only minor changes are requested by the working group or Task Force, the Working Group Chair may be the only one to re-review and approve the subsequent draft document.
b,c Following list approval, invited clinical experts, stakeholders and peer reviewers will have declared interests assessed by the Task Force Conflict of Interest Oversight Committee.
dApproval by non-objection: approved unless Task Force members communicate any objections
eApproval by consensus: agreement by at least two-thirds of members (irrespective of presence or absence in decision-making meeting)
fFull approval: an email from each of the working group or Task Force members indicating their approval
1.7.2.1 Addressing objections raised during non-objection and full approval processes
In the case that objections are raised during an approval by non-objection or full approval, the group seeking approval of the document will work to address the concerns raised and recirculate to the working group or full Task Force for another round of approval. In the case that an impasse is reached (e.g., one or more Task Force members object to approval of a document despite several rounds of review and revision), the issues giving rise to the objection can be brought to a blinded vote (e.g., using anonymized voting software), for which the voting rules described above will apply (i.e., if 2/3 of Task Force members agree on a particular direction, the issue giving rise to the objection will be considered resolved). Upon resolution of issues giving rise to objection(s), the document will be considered approved and will move forward to the next stage of review or submission. All Task Force members eligible for authorship are expected to participate as authors on the document to reflect their participation in the process.
1.8 Conflicts of Interest and Confidentiality
1.8.1 Conflicts of Interest
The Task Force policy on disclosures of interests and management of conflicts of interest can be found on the Task Force Website (https://canadiantaskforce.ca/wp-content/uploads/2020/10/COI-Policy-202008Final-1.pdf). Guidance in this policy applies to all current and prospective Task Force members. The policy also includes an overview of procedures followed for groups that provide support to the Task Force, including invited clinical or content experts, external stakeholders and peer reviewers, Evidence Review and Synthesis Centres, the Science Team at PHAC and the KT Team.
1.8.2 Confidentiality Agreements
1.8.2.1 Canadian Task Force on Preventive Health Care Members
Task Force members are required to sign Confidentiality Agreements (Appendix 1) before commencing membership on the Task Force. Members are expected to hold in confidence, and not disclose, any confidential information, as defined in the Confidentiality Agreement, during their membership on the Task Force. Members will not publicly release information on the deliberations of the Task Force or information obtained in the course of these deliberations until the information has been published. Completed forms are filed and reviewed by the Task Force office.
1.8.2.2 Science Team at the Public Health Agency of Canada
Science Team staff follow the Public Service Values and Ethics Code (6) and manage information in accordance with the Access to Information Act (7) and Privacy Act (8).
1.8.2.3 Evidence Research and Synthesis Centres, Knowledge Translation Team and Communications Team
Staff from the ERSCs, KT Team and Communications Team sign a Confidentiality Agreement every two years they are involved with the Task Force. Staff are expected to keep all the documents and information as defined in the Confidentiality Agreement strictly confidential, not use any Confidential Information for any purpose other than those indicated by the Task Force and not disclose any Confidential Information to any third party without the prior written consent. Forms completed by the ERSC and KT Team members are saved on their institutional servers and forms completed by the Communications Team are filed by the Task Force Office.
1.8.2.4 External Partners and Reviewers
Upon joining a project and every two years they are involved with the Task Force, clinical or content experts, stakeholders and peer reviewers will sign a Confidentiality Agreement (Appendix 2), whereby they acknowledge that all documents and information received from the Task Force, or that may be developed while working on a Task Force review or guideline, are strictly confidential and shall not be disclosed to any third party without the prior written consent of the Task Force. Other external partners who review pre-publication Task Force documents will be asked to sign a Confidentiality Agreement (e.g., partners who review draft guideline for endorsement). Completed forms are reviewed and filed by the Task Force office.
1.9 Public and Social Media Activities
Task Force members are encouraged to discuss, disseminate, and defend the published recommendations of the Task Force in public fora (e.g. social media platforms like Twitter and Instagram), as advised by the Task Force Communications Team.
1.9.1 Dealing with the Media
The Chair, Vice-Chair, or other members of the Task Force may make comments or statements to the media or on social media at the discretion of the Chair and Vice-Chair and with advice from the Communications Team. The Communications Team manages the Task Force’s social media channels (Twitter, Instagram, LinkedIn and Facebook).
1.10 Authorship
Authorship for journal articles or other documents for public dissemination is assigned in accordance with the recommendations of the International Committee of Medical Journal Editors (http://www.icmje.org). In addition, all members of the Task Force at the time of voting on guideline recommendations will be identified as individual authors in the publication (unless excluded from voting due to reasons of conflict of interest). All Task Force members eligible for authorship are expected to participate as authors (irrespective of view on final recommendations) to reflect their participation in the guideline process. Further details on authorship criteria are provided in Chapter 4 for protocols and evidence reviews and in Chapter 5 for guidelines.
1.11 Overview of the Guideline Development Process
The Task Force’s guideline development process is guided by the GIN-McMaster Guideline Development Checklist (9,10). The Task Force follows the GRADE approach as outlined in the GRADE Handbook (3) and a series of articles in the Journal of Clinical Epidemiology (11) for rating the certainty of evidence and grading the strength of recommendations.
Once a research topic is identified and prioritized by the Topics Working Group for a possible guideline, a guideline working group of Task Force members is formed and an ERSC is assigned. External linkages with clinical/content experts, stakeholders, and peer reviewers are made, and work begins on the guideline process. A scoping document prepared by the Science Team provides background information for the members of the working group and helps them to define the key questions and scope (e.g., population(s), intervention(s), comparator(s) and outcomes). A protocol for the evidence reviews that will support guideline development is prepared, the evidence reviews are conducted by the ERSC. Guideline recommendations are developed by members of the working group in conjunction with members of the full Task Force. Functional working groups, with responsibility for topic prioritization, methods, knowledge translation, and equity support these processes (see Section 1.6.2 for roles of these working groups).
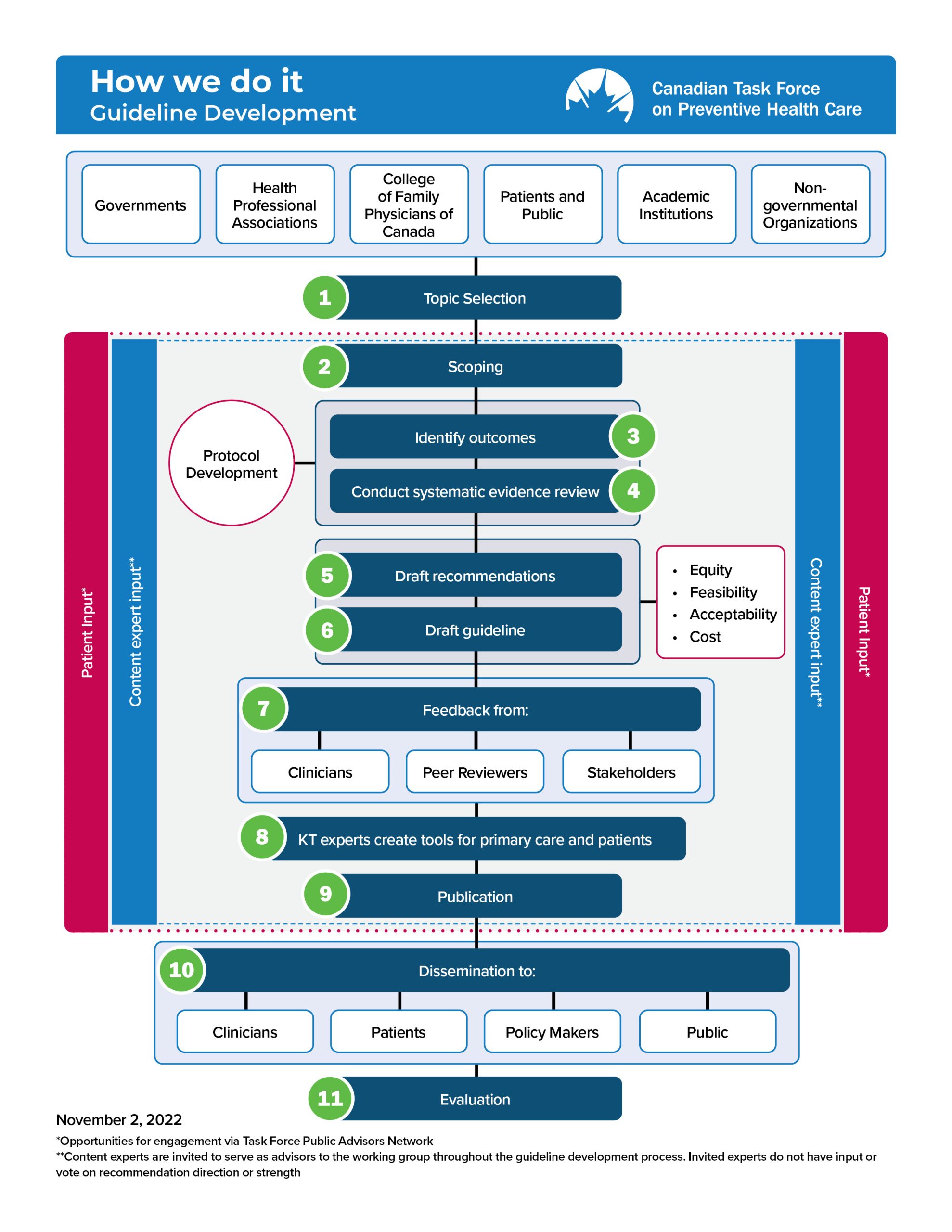
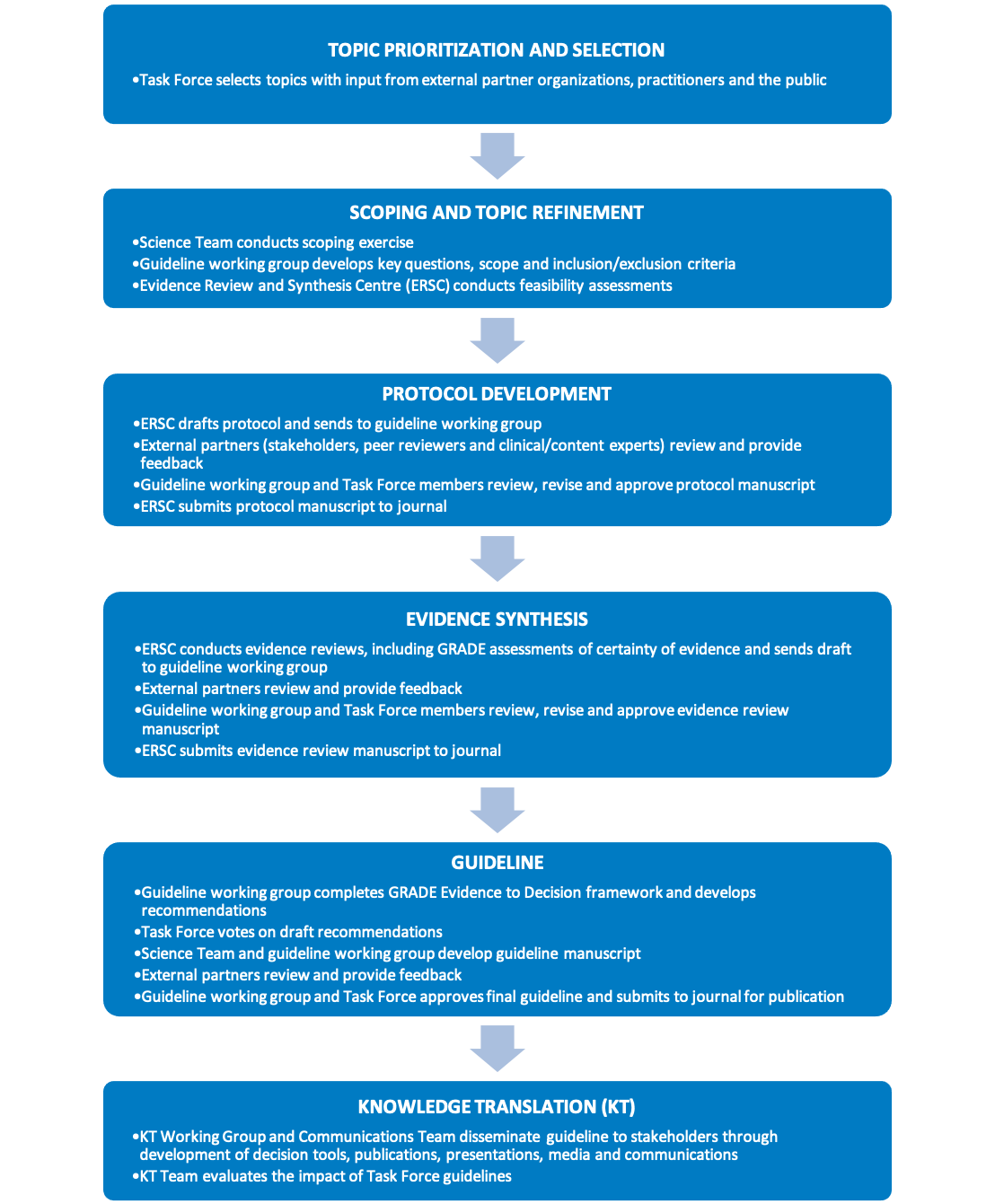
Figure 1 provides an overview of the Task Force’s methodology. The key elements of the guideline development process are shown in Figure 2. These processes are further described in the following chapters.
Figure 1. Overview of Task Force methodology
Figure 2. Key elements of the Task Force guideline development process
References
- Atun R. What are the advantages and disadvantages of restructuring a health care system to be more focused on primary care services? Copenhagen, WHO Regional Office for Europe (Health Evidence Network report) [Internet]. 2004 [cited 2020 Jan 30]. Available from: http://www.euro.who.int/__data/assets/pdf_file/0004/74704/E82997.pdf
- Last J. A dictionary of public health. Oxford: Oxford University Press; 2007.
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook [Internet]. 2013 [cited 2021 Oct 25]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html
- U.S. Preventive Services Task Force. Procedure Manual [Internet]. 2021 [cited 2021 Sep 21]. Available from: https://www.uspreventiveservicestaskforce.org/Page/Name/procedure-manual
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021) [Internet]. 2021 [cited 2021 Oct 13]. Available from: https://training.cochrane.org/handbook
- Government of Canada. Values and Ethics Code for the Public Sector [Internet]. 2011 [cited 2022 Jan 6]. Available from: https://www.tbs-sct.gc.ca/pol/doc-eng.aspx?id=25049
- Government of Canada. Access to Information Act (R.S.C., 1985, c. A-1) [Internet]. 2021 [cited 2022 Jan 6]. Available from: https://laws-lois.justice.gc.ca/eng/acts/A-1/index.html
- Government of Canada. Privacy Act (R.S.C., 1985, c. P-21) [Internet]. 2021 [cited 2022 Jan 6]. Available from: https://laws-lois.justice.gc.ca/eng/acts/p-21/index.html
- Schünemann HJ, Wiercioch W, Etxeandia I, Falavigna M, Santesso N, Mustafa R, et al. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. Can Med Assoc J [Internet]. 2014 Feb 18;186(3):E123 LP-E142. Available from: http://www.cmaj.ca/content/186/3/E123.abstract
- McMaster University. GIN-McMaster Guideline Development Checklist [Internet]. [cited 2021 Sep 21]. Available from: https://cebgrade.mcmaster.ca/guidecheck.html
- Journal of Clinical Epidemiology. GRADE Series [Internet]. 2021 [cited 2021 Sep 21]. Available from: https://www.jclinepi.com/content/jce-GRADE-Series
Appendices
Appendix 1: Confidentiality Agreement for Task Force Members