Chapter 2: Topic Prioritization, Surveillance, Review, and Selection
Last updated: November 28, 2023
2 Topic Prioritization, Surveillance, Review, and Selection
The Task Force selects new topics for guideline development and/or updates of existing guidelines in a multistage process that includes input from practitioners, external stakeholders and the public. The Task Force solicits new topics to develop guidelines for screening and prevention interventions in primary care through the Task Force website which is accessible to any interested party. The Task Force may also solicit topic suggestions from the College of Family Physicians of Canada and other key stakeholders (e.g., governmental and non-governmental organizations, patient advocacy groups, and professional associations). If the Topics Working Group decides that it is necessary (e.g., due to limited topic suggestions or updates), the Task Force may perform a horizon scan to determine if there is any new evidence, interventions, methods or hot topics relevant to preventive health to be considered for new topics. All published guidelines undergo ongoing surveillance to inform if an early update should be considered (e.g., change in practice, new trial evidence). At the 5-year mark from publication, guidelines are reviewed to determine if they should be updated, reaffirmed or sunsetted.
2.1 Topic Identification
The Science Team supports the Task Force topic prioritization and selection process by completing several linked activities to gather an initial list of topics for consideration.
2.1.1 New Topics
2.1.1.1 Topic Submission via the Task Force Website
Suggestions for topics are solicited from the public via the Task Force website (https://canadiantaskforce.ca/submit-topic-suggestions/). Anyone can submit a topic by clicking on “Get Involved” >> “Submit Topic Suggestions” and using the provided form.
2.1.1.2 Open Call for Topics
To solicit additional suggestions for new topics, an open call email may be sent to a list of key stakeholders and all current Task Force members. Stakeholders, such as the Canadian Medical Association and the College of Family Physicians of Canada, would be invited to complete the online topic submission form (as referenced above).
2.1.1.3 Previously Short-listed New Topics
New topics which were previously short-listed (see section 2.4.1) but were not selected for guideline development may be reconsidered in the next submission cycle. Previously short-listed new topics must have support from at least one Task Force member to continue to be considered during the prioritization process. Topics that were previously not selected during the Delphi process (see section 2.4.3) will remain on the list of previously short-listed topics for a maximum of 3 selection cycles (i.e., 3 years) at which time they will be removed from the short list.
2.1.1.4 Horizon Scan
Depending on need, a horizon scan may be completed to identify emerging issues. The scan may include assessments of grey literature (e.g., news media, bibliographic databases, premier journals, and new or upcoming guidelines from other leading guideline development organizations (e.g., United States Preventive Services Task Force, UK National Screening Committee), and review of the Canadian Agency for Drugs and Technology in Health (CADTH) Horizon Scan (1) which scans for new and emerging health technologies relevant to the Canadian health care system.
2.1.2 Guideline Updates
2.1.2.1 Early Updates (Ongoing Surveillance)
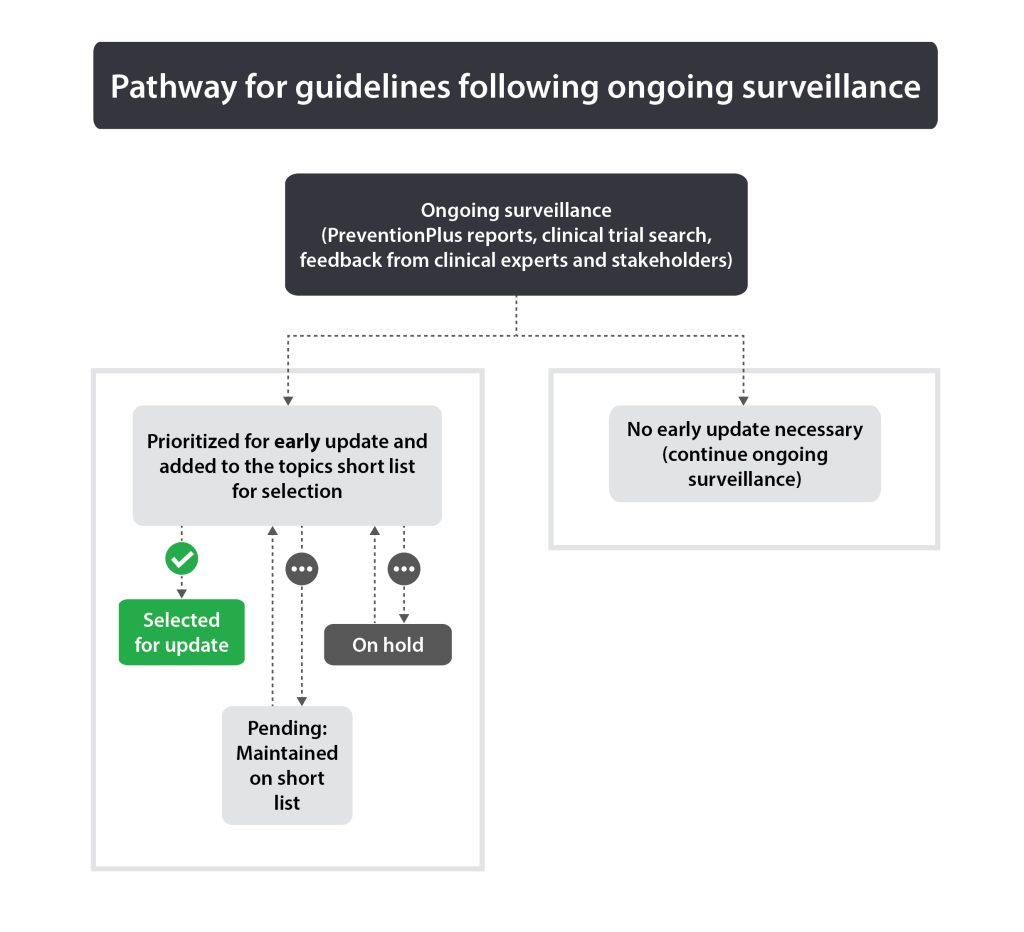
Guidelines published less than 5 years ago with important new evidence or significant changes in practice may warrant an early update. If ongoing surveillance identifies this (see section 2.3.1), the guideline will be added to the topics short list.
2.1.2.2 Updates (Five-year Review Timeline)
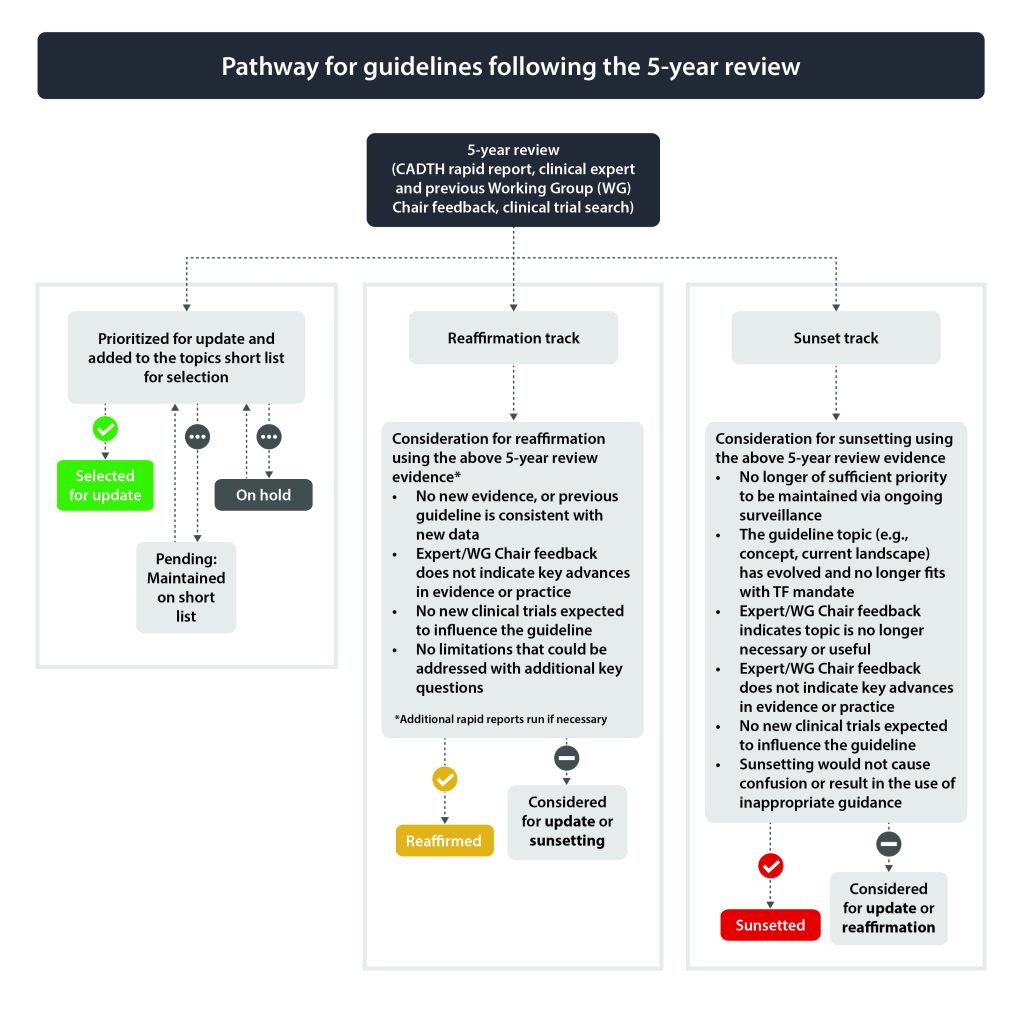
Guidelines due for 5-year review (see section 2.3.2) will be assessed by the Topics Working Group for update, reaffirmation or sunsetting. Those selected for potential update will be added to the topics short list. See section 2.3.2.2 (reaffirmation) and 2.3.2.3 (sunsetting) for options for topics not selected for potential update.
2.1.2.3 Pending Guideline Updates
Guidelines that are selected as candidates for update (early or at 5 years) are placed on the topics short list (see section 2.4.1). However, if they are not selected during the final Delphi round, they will remain on the short list as “pending” and will be reconsidered in the next topic selection round. Additionally, guidelines that enter the reaffirmation or sunsetting process but fail to meet the criteria will be considered as pending until they are resolved as either updates, reaffirmations or sunsetted guidelines. Guidelines should not stay in the “pending” category for more than 3 years following the 5-year review process. At the 3-year point (following the 5-year review), if existing guidelines have not been reaffirmed or updated, they will be sunsetted.
2.1.2.4 “On Hold” Guideline Updates
Guidelines that are deemed out of date but cannot be addressed at this point in time are considered to be “on hold.” Guideline updates may be on hold due to a need to reassess how the Task Force should address the update or due to the changing landscape of the subject matter. Unlike pending updates, “on hold” guideline updates are not placed on the topics short list until they have been reassessed by the Task Force. In these situations, a hold statement will be posted on the Task Force website to alert health care professionals and the public that the guidelines are out of date.
2.2 New Topic Review and Preparation of Brief Assessments
Each topic suggestion from the horizon scan and online submissions will be reviewed by two Topics Working Group members to assess whether the topic fits within the mandate of the Task Force. Those that clearly do not fit the mandate will be removed. Conflicts are resolved by Topics Working Group consensus or voting if necessary. Once this review is completed, the Science Team will conduct a brief assessment of each topic which includes:
- Assessment of concordance with the mandate of the Task Force to ensure relevance.
- Results of a brief scan for relevant existing guidelines on the topic.
- Assessment of impact for new topic guidelines based on 4 broad criteria:
- Is this an area where there is a screening or prevention intervention that is not commonly provided but may be beneficial?
- Is this a screening or prevention intervention that is commonly provided but may not be justified if benefits, harms, and resources are carefully evaluated?
- Is this an area where there is controversy about the benefits or harms of the intervention?
- General assessment using the Wilson and Jungner screening criteria (2,3):
- Knowledge of disease:
- The condition should be important.
- There must be a recognisable latent or early symptomatic stage.
- The natural course of the condition, including development from latent to declared disease, should be adequately understood.
- Knowledge of test:
- Suitable test or examination.
- Test acceptable to population.
- Case finding should be continuous (not just a ‘once and for all’ project).
- Treatment for disease:
- Accepted treatment for patients with recognised disease.
- Facilities for diagnosis and treatment available.
- Agreed policy concerning whom to treat as patients.
- Cost considerations:
- Costs of case finding (including diagnosis and treatment of patients diagnosed) are economically balanced in relation to possible expenditures on medical care as a whole.
- Knowledge of disease:
2.3 Guideline Surveillance and Review
2.3.1 Ongoing Surveillance Process
The purpose of ongoing surveillance is to alert the Task Force to significant new evidence that may lead to a decision to update a guideline earlier than anticipated. Each published guideline undergoes continuous surveillance via PreventionPlus (evidence alerts) (4), review of clinical trials and ongoing feedback from clinical experts and key stakeholders. The threshold for “significant” new evidence varies by guideline, as strong recommendations based on multiple trials with high certainty data may require substantive new contrary evidence compared to guidelines with conditional recommendations and/or those with low certainty or limited data. Input from the guideline’s Working Group Chair (or member if unavailable) on evidence thresholds may be requested. The Science Team reviews the evidence continuously and creates monthly summaries. The Topics Working Group will review the collected evidence annually (or earlier if deemed necessary by the Science Team and Topics Working Group Chair) to determine if a guideline should be considered for early update or continue ongoing surveillance.
Any guideline that is identified for possible early update by the Topics Working Group will be put onto the topics short list. See Figure 1 for pathways for guidelines following ongoing surveillance.
2.3.2 Five-year Review Process
Guidelines due for their 5-year update will undergo a more rigorous review to determine if they should be considered for a full update, reaffirmation or sunsetting (i.e., archive guideline).
The 5-year review plan involves:
- Review of the previous ongoing surveillance evidence (see section 2.3.1).
- CADTH rapid response report(s) (5)
- Reference and abstract search on relevant key questions (relevant key questions to be determined based on input from clinical experts involved in the previous guideline and prior Working Group Chairs or member).
- Critical appraisal of articles if necessary.
- Clinical trial registry search (https://clinicaltrials.gov, https://trialsearch.who.int/, https://www.clinicaltrialsregister.eu/, https://www.isrctn.com/)
- Solicited feedback from clinical expert(s).
- Solicited feedback from previous Working Group Chair (or member).
The Topics Working Group reviews the collected evidence to determine if the guideline should be considered as a candidate for (a) full update, (b) reaffirmation or (c) sunsetting (i.e., archive guideline). See Figure 2 for pathways for guidelines following the 5-year review.
2.3.2.1 Updating Process
Guidelines selected for potential update (early or at 5 years) will be put onto the topics short list during the prioritization process (see section 2.4.1).
2.3.2.2 Considerations and Process for Reaffirmation
Reaffirmation is an efficient way of reviewing and endorsing an existing Task Force guideline when a full systematic review and update is deemed unnecessary. Reaffirmation also communicates the continued relevance of a guideline to Task Force stakeholders, including primary care clinicians that apply guidelines regularly to practice.
Benefits of reaffirmation:
- Reduces the backlog of full guideline updates and saves resources.
- Keeps guidelines up-to-date and relevant without a full evidence review update or GRADE assessment of new evidence that is deemed unlikely to change the guideline.
- Allows for re-endorsement from stakeholder organizations (provides background and rationale).
- Important for clinicians, to support confidence in guidelines more than 5 years old.
- Indicates to stakeholders that the Task Force continues to value and prioritize the guideline.
2.3.2.2.1 Reaffirmation Methods
Guidelines that have been selected for consideration for reaffirmation in the 5-year review process (see section 2.3.2) undergo the following steps:
- The Science Team will summarize the previous guideline (title, publication year, recommendation(s), methods, findings, conclusions).
- The Science Team will assess the accumulated evidence (see section 2.3.2) since the previous publication search date.
- The Topics Working Group will review and weigh the evidence based on the following considerations for reaffirmation:
- Is there new evidence for this guideline?
- Is the new evidence consistent with the previous guideline (e.g., direction and strength of the recommendation)?
- Does feedback from clinical experts and Working Group Chairs or members indicate key advances in evidence or practice in this area since the guideline was published? This may include changes to healthcare models, patient management, regulatory changes, equity, feasibility, patient values and preferences, acceptability or costs that might impact the guideline.
- Are there relevant clinical trials that are expected to be completed within the next few years?
- Are there any unaddressed gaps or limitations in the previous guideline that could be improved with additional key questions or changes to key aspects (e.g., population(s), intervention(s), comparator(s), outcome(s), timing, setting(s))?
- The Topics Working Group will make a recommendation to the Task Force on whether or not the guideline should be reaffirmed.
- The Task Force will review the evidence and rationale and make a decision on whether to approve reaffirmation of the guideline. If the guideline is not reaffirmed, it will return to the Topics Working Group for consideration for potential update or sunsetting.
- Communication
- If reaffirmation is deemed appropriate, a brief summary, rationale and recommendation statement will be posted on the Task Force website. It will also indicate that each reaffirmed guideline will continue to undergo ongoing surveillance for new evidence.
- The Task Force will alert key stakeholders of their decision to confirm.
- If endorsement for a reaffirmed guideline is sought, the endorsing partner will be provided with sections 1-5 above, as well as a brief summary for the decision, and will be informed that the guideline will continue to undergo ongoing surveillance and re-evaluation.
Reaffirmation methods do not include a GRADE assessment incorporating any new evidence. Therefore, the reaffirmation statement will not comment on the certainty of the evidence beyond what was found in the original guideline. If a guideline is reaffirmed, the Task Force will provide a clear rationale showing that the direction (for/against) and strength (conditional/strong) of the recommendation has not changed.
2.3.2.3 Considerations and Process for Sunsetting
Guidelines that have been selected for consideration for sunsetting during the 5-year review process, or following a decision not to confirm or update, will undergo the following steps:
- The Science Team will summarize the previous guideline (title, publication year, guideline(s), methods, findings, conclusions).
- The Science Team will assess the accumulated evidence (see section 2.3.2) since publication in relation to previous guideline(s).
- The Topics Working Group will review the evidence and compare it against the considerations for sunsetting:
- Is the guideline no longer relevant to primary care in Canada?
- Has the guideline topic (e.g., concept, current landscape) evolved and no longer fits with TF mandate?
- Does feedback from clinical experts or Working Group Chair or members indicate that the guideline is no longer necessary or useful?
- Are there any new changes to healthcare models, patient management, regulatory changes, equity, feasibility, patient values and preferences, acceptability or costs since the guideline was published?
- Is the guideline no longer of sufficient priority to be maintained via ongoing surveillance?
- Do other current Canadian guidelines align with Task Force guidelines (i.e., sunsetting would not cause confusion or result in the use of inappropriate guidance)?
- The Topics Working Group will make a recommendation to the Task Force on whether or not the guideline should be sunsetted.
- The Task Force will review the evidence and rationale and make a decision on whether to approve sunsetting of the guideline. If the guideline is not sunsetted, it will return to the Topics Working Group for consideration for potential update or reaffirmation.
- Communication: If sunsetting is deemed appropriate a brief summary, rationale and statement will be posted on the Task Force website.
For a full description of the reaffirmation and sunsetting methods, please click here.
2.4 Topic Selection
2.4.1 Development of Topics Short List
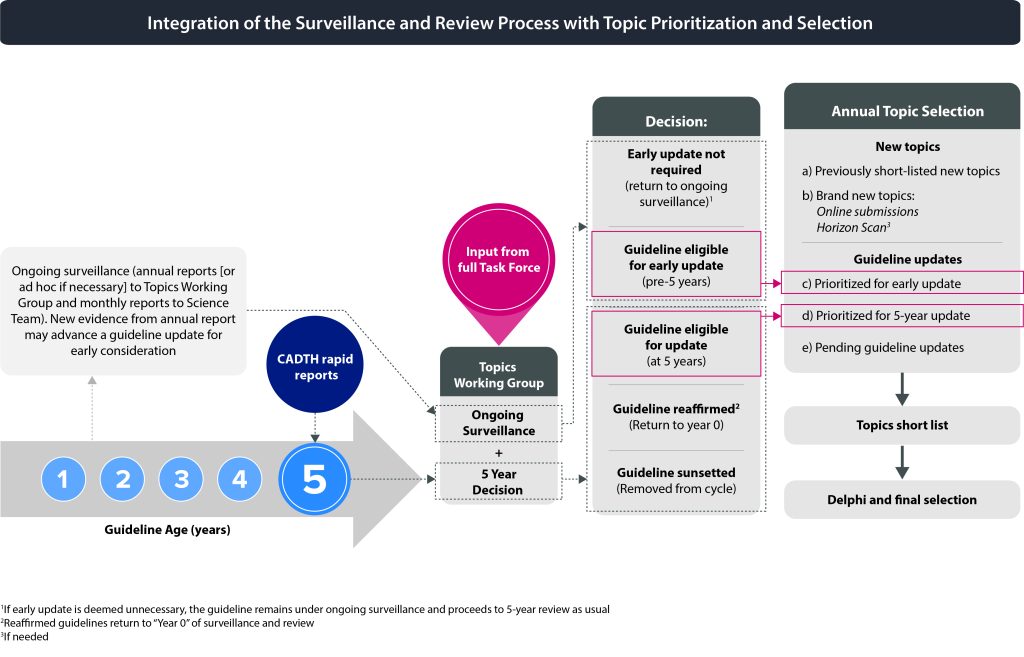
The Topics Working Group will meet to consider the brief assessments of each topic on the initial list (see section 2.2) with a goal of using discussion and consensus to create a short list of topics for further development. The Topics Working Group will consider the potential impact of each topic (See section 2.2) as well as how many topics will be actioned for the following year in their assessment (e.g., does the topic fill a gap area in Task Force guidance). The impact can also be influenced by burden of disease, uncertainty in practice, or availability of evidence. The selected candidate topics from the horizon scan, and online submissions will be combined with the previously short-listed topics, early updates, 5-year updates and pending updates to create a final short list of topics. The short list should include approximately 5-7 topics and will be approved by the Topics Working Group. The short list is presented to the full Task Force for approval.
2.4.2 Preparation of Topic Briefs
A topic brief will be developed by the Science Team for each topic short-listed by the Topics Working Group. This document (2-3 pages) provides Task Force members with the information required for further topic prioritization and selection. The topic brief is informed by the brief assessment (for new topics), CADTH rapid reports (for guideline updates) or previous guideline (for guideline updates). Additionally, the Science Team will carry out a brief search in guideline databases/websites (e.g., Canadian Medical Association Infobase, Guidelines International Network, Scottish Intercollegiate Guidelines Network) and summarize their recommendations. The Science Team will also search bibliographic databases (e.g., Cochrane, PubMed) for systematic reviews and randomized controlled trials on each topic. The topic brief also includes details on the following items:
Brief background on the issue including burden of disease in Canada (incidence, prevalence, morbidity, mortality, co-morbidity, and quality of life).Relevance of the topic to the mandate of the Task Force (new topics only).
- Brief background on the issue including burden of disease in Canada (incidence, prevalence, morbidity, mortality, co-morbidity, and quality of life).
- Relevance of the topic to the mandate of the Task Force (new topics only).
- Is this an area where there is a screening or prevention intervention that is not commonly provided but may be beneficial (e.g., information on current practice, prevention intervention characteristics in Canada; new topics only)?
- Is this a screening or prevention intervention that is commonly provided but may not be justified if benefits, harms, and resources are carefully evaluated (e.g., recent controversy, lack of evidence)?
- Is this an area where there is uncertainty about the balance of benefits and harms of the intervention?
- Is there noted variation in practice in Canada or variation in health outcomes (e.g., variation in provincial guideline recommendations, new recommendations)?
- The screening or preventive strategy (i.e., intervention; new topics only).
- Potential impact of the screening or preventive strategy (new topics only).
- New evidence that has the potential to change prior recommendations (for updates only).
- Sufficiency of the evidence (i.e., anticipated amount of available evidence).
- New or upcoming guidelines or systematic reviews.
2.4.3 Delphi Process
The intention of the final phase of topic selection is to provide the Task Force with an opportunity to review priority topics and to make an informed decision on which new topics or updates should be initiated.
As described by Gorber and colleagues (2012), the final topic selection process will involve a formal Delphi process with the Task Force (6). Members of the Task Force will be asked to review all the topic briefs and indicate their assessment of the priority of each short-listed topic (high to low priority) on the health status of Canadians (Delphi round 1). Impact is defined as whether a guideline on this topic has the potential to change practice and improve the health of Canadians and our health care system. The results of round 1 will be collated and recirculated to the Task Force to help prepare for Delphi round 2. The Task Force will again rate the priority of the short-listed topics during the second Delphi round.
The Science Team will work with the members of the Topics Working Group to develop a presentation of the results from rounds 1 and 2 for members of the Task Force for final topic selection.
2.4.4 Final Topic Selection
The Task Force will be presented with the results of rounds 1 and 2 of the Delphi process for their consideration. Task Force members will discuss the ratings and select the final topics based on a Delphi consensus process. The number of guidelines to be initiated each year may vary depending on available resources. Reaffirmation topics do not require the same amount of resources as updates and will be considered separately from the topic selection process (i.e., not part of the Delphi process). Figure 3 shows how the surveillance and review process is integrated with topic prioritization and selection.
References
- Canada’s Drug and Health Technology Agency. Horizon Scan [Internet]. 2022 [cited 2022 Nov 24]. Available from: https://www.cadth.ca/horizon-scan
- Harding M, Jackson C. Screening Programmes in the UK, Wilson and Jungner criteria for screening [Internet]. 2014 [cited 2021 Oct 1]. Available from: https://patient.info/doctor/screening-programmes-in-the-uk#nav-0
- Wilson JMG, Jungner G. Principles and Practice of Screening for Disease. World Health Organization; 1968.
- McMaster University. PREVENTIONPlus [Internet]. 2022 [cited 2022 Jun 17]. Available from: https://plus.mcmaster.ca/PreventionPlus/
- Canadian Agency for Drugs and Technologies in Health. About the Rapid Response Service [Internet]. 2011 [cited 2021 Oct 1]. Available from: https://www.cadth.ca/about-rapid-response-service
- Gorber SC, Singh H, Pottie K, Jaramillo A, Tonelli M. Process for guideline development by the reconstituted Canadian Task Force on Preventive Health Care. CMAJ. 2012 Oct;184(14):1575–81.