Chapter 3: Initiating the Guideline Development Process for Selected Topics
Last updated: March 30, 2023
3 Initiating the Guideline Development Process for Selected Topics
3.1 Overview of the Scoping Exercise
3.3 Presentation of Scoping Information
3.5 Key Questions on Intervention Effects
3.5.1 Defining the Patients or Population
3 Initiating the Guideline Development Process for Selected Topics
The Task Force selects new topics for guideline development and/or updates of existing guidelines in a multistage process that includes input from practitioners, external stakeholders and the public. The Task Force solicits new topics to develop guidelines for screening and prevention interventions in primary care through the Task Force website which is accessible to any interested party. The Task Force may also solicit topic suggestions from the College of Family Physicians of Canada and other key stakeholders (e.g., governmental and non-governmental organizations, patient advocacy groups, and professional associations). If the Topics Working Group decides that it is necessary (e.g., due to limited topic suggestions or updates), the Task Force may perform a horizon scan to determine if there is any new evidence, interventions, methods or hot topics relevant to preventive health to be considered for new topics. All published guidelines undergo ongoing surveillance to inform if an early update should be considered (e.g., change in practice, new trial evidence). At the 5-year mark from publication, guidelines are reviewed to determine if they should be updated, confirmed or sunsetted.
Confirmation methods do not include a GRADE assessment incorporating any new evidence. Therefore, the confirmation statement will not comment on the certainty of the evidence beyond what was found in the original guideline. If a guideline is confirmed, the Task Force will provide a clear rationale showing that the direction (for/against) and strength (conditional/strong) of the recommendation has not changed.
3.1 Overview of the Scoping Exercise
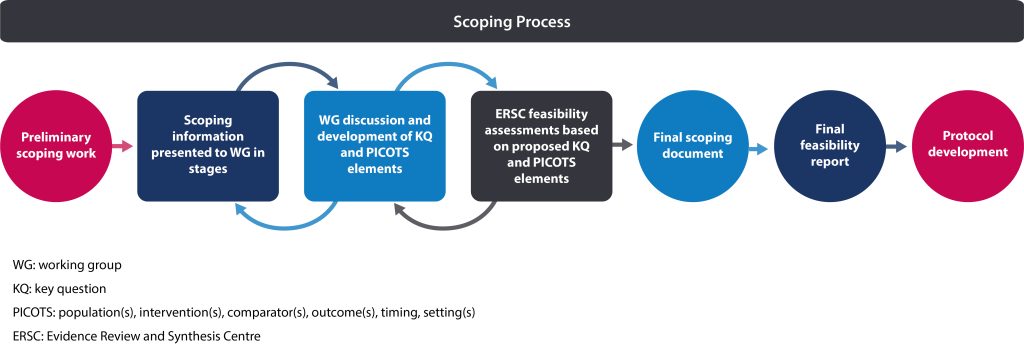
Following selection of a topic (see section 2.4.4) and formation of the guideline working group (see section 1.6.1), a scoping exercise is conducted. This exercise provides the working group with additional information to consider as they refine the topic prior to protocol development. The scoping exercise is not a formal scoping review; it is meant to provide the working group with necessary information needed to inform and develop the protocol. The Science Team builds on the topic brief (see section 2.4.2) by conducting a preliminary scoping search to identify key literature. Information is presented to the working group in stages as they discuss and formulate each possible key question (KQ) and aspects of each possible KQ such as population(s), intervention(s), comparator(s) and outcome(s), timing and setting(s) (PICOTS*) for the protocol. In addition, the Evidence Review and Synthesis Centre (ERSC) may conduct feasibility assessments of draft KQs for the working group’s consideration.
The outputs of this scoping exercise are:
- Final scoping document (prepared by the Science Team)
The final scoping document provides an overview of the evidence and a general framework from which to establish what the guideline will include and what will not be covered. This document is used by the working group and ERSC to form the basis of the protocol.
- Final feasibility report (prepared by the ERSC)
The final feasibility report specifies the search strategy, budget and timelines for each KQ and the overall work conducted by the ERSC. This report is reviewed and approved by the Working Group Chair, Science Team Lead and Science Team Manager before moving forward to the protocol stage.
Table 1 outlines key differences between the brief assessment, topic brief, scoping document, feasibility report and protocol. Figure 1 provides an overview of the process from the initial scoping search to protocol development.
*The PICOTS format is not the only approach to specifying details of the key questions; however, it will be used throughout this chapter as it is the format most commonly used by the Task Force.
Table 1. Differences between brief assessment, topic brief, scoping document, feasibility report and protocol
| Brief Assessment | Topic Brief | Scoping Document | Feasibility Report | Protocol |
|
|
|
|
|
3.2 Preliminary Scoping Work
The Science Team will locate and summarize existing and recent (<5 years) relevant international (but at national level within) and national/provincial/regional Canadian guidelines from other organizations relevant to the topic (e.g., screening, prevention, management) with a specific focus on those underpinned by systematic review evidence. The Science Team will identify whether recommendations differ among guidelines and how they differ (e.g., differing PICOTS, evidence used (including study designs), value judgements such as rating of outcomes, cost-effectiveness). The Science Team will also comment on use of GRADE or other quality appraisal, limitations of evidence and other considerations including feasibility, acceptability, cost and equity issues from guideline panels.
The Science Team will conduct a search for recent (<5-10 years) systematic reviews relevant to the topic (may include natural history [including different prognosis for specific populations], screening, prevention, treatment, test accuracy, risk assessment, patient preferences), then screen, select and summarize this information. In addition, the Science Team will search for Canadian statistics on burden (e.g., incidence, prevalence, trends over time, cost of disease).
In the case of updates to existing Task Force guidelines, the Science Team will provide background on new evidence (e.g., CADTH rapid reports) since the original guideline’s evidence review, how patient values and preferences were determined in the original guideline, assessment of any major changes in the intervention due to technological or other developments as well as an assessment of any methodological gaps in the development of the original guideline. The members of the working group use this information to determine if the scope of the guideline will change as part of the update.
Information produced by the Science Team as part of this scoping search will be presented to the working group in stages, as described in section 3.3.
3.3 Presentation of Scoping Information
The scoping document is a living document throughout early working group discussions. Generally, information is presented in stages to allow the working group to focus and refine each element of the guideline (e.g., PICOTS) separately and then build on this information to complete the scope. Some elements may require more or less refinement based on what was identified at the topic brief stage (e.g., in some cases the overall population of interest may be immediately evident). In some cases, it will be helpful for PICOTS elements from different KQs to be discussed at the same time (e.g., when screening and treatment questions result in similar patient important outcomes). It is important for the working group to have early discussions about the full prevention pathway including indirect evidence that might be necessary to inform recommendations (e.g., diagnostic test accuracy, treatment following diagnosis, overdiagnosis).
The Science Team will summarize information relevant to each PICOTS element (e.g., comparison between guidelines/reviews) in stages and document decisions made when each element is discussed and finalized. For example, when the working group finalizes their decision on the population of interest, this section of the scoping document is finalized, and the rationale drawn from discussions and decisions of the working group is documented. Rationale for inclusion and exclusion criteria should be well-supported with clinical or other rationale.
3.4 Key Questions
The Task Force develops key questions for each guideline; they are formulated to direct the evidence review and inform the guideline recommendations. At a minimum, each Task Force guideline will include a main KQ on the benefits and harms of the target screening or preventive intervention(s). Methods for developing such intervention KQs are described in section 3.5.
Additional KQs may also be related to the main intervention, when evidence on the direct effects of the intervention (e.g., screening vs. no screening) is not anticipated to be sufficient to make a recommendation. For example, KQs on the accuracy of the screening tests and/or the effects of the related treatment may be required to enable indirect inferences about the main intervention effects. Moreover, KQs related to other domains in the Evidence-to-Decision (EtD) framework (1,2) (e.g., patient preferences, acceptability of intervention for patients and/or clinicians, cost-effectiveness) may be added when they are considered highly informative for decisional uncertainty around the guideline topic. Further details on non-intervention KQs are provided in section 3.6.
Key questions for an updated review (e.g., when updating a previous guideline) may focus on a limited aspect of the topic and may be used to examine gaps in the evidence for the previous review or to examine new evidence published since the previous review. For example, the guideline working group may decide to focus on only certain interventions if there was moderate or high certainty others were not effective. The working group may also decide to only examine comparative effectiveness if effects versus usual care are certain and fully implementable in practice.
3.5 Key Questions on Intervention Effects
Each Task Force guideline includes a KQ on effectiveness of screening or prevention interventions. Framing these KQs involves carefully specifying the relevant characteristics using a PICOTS approach (3,4) which is critical to conducting the evidence review and developing recommendations.
These are defined as:
Population: the patients or population to whom the recommendations are meant to apply
Intervention: the screening or prevention intervention(s) under investigation
Comparison: the alternative intervention(s); could be usual care, no intervention or another intervention
Outcome: the patient-important outcomes defined as critical or important to decision-making (may be desirable [i.e., benefit] or undesirable [i.e., harm])
Timing: the timing of outcome assessment (e.g., duration of follow-up, single or multiple follow-up assessments)
Setting: the setting in which the guideline will be implemented
The members of the working group determine what PICOTS elements should be included in the review and which should be excluded. Study designs of interest may not be known at this stage; if they are known they may be addressed in the PICOTS table (3).
The guideline recommendation question determines corresponding KQs for the evidence review:
- Recommendation question: Should [intervention] vs. [comparison/usual care] be used for [health problem or population]?
- Key question: What are the benefits and harms of [intervention] versus [comparison/usual care] for [health problem or population] (3).
The KQs are directly linked with the analytic framework (section 3.5.6) and serve to focus the evidence review. They specify the PICOTS elements for the topic under consideration and are critical to conducting the evidence review to support development of recommendations.
Questions on the appropriate interval for screening interventions and special considerations for particular populations may also be included as appropriate. Separate evidence summaries may need to be produced for specific populations at different baseline risk of an outcome and those for whom certainty of evidence may differ (5).
3.5.1 Defining the Patients or Population
The characteristics of the patients or population who will be the focus of the recommendation(s) are identified; this may include their sex, age, location and setting in which they access care, among other characteristics.
Equity considerations highlighted by the PROGRESS-Plus (6,7) framework will also be considered in defining the overall population and potentially disadvantaged groups within the broader population. They include:
- Place of residence
- Race/ethnicity/culture/language
- Occupation
- Gender
- Sex
- Religion
- Education
- Socioeconomic status
- Social capital
Populations may be excluded from scope of the guideline by consensus of the working group if they would not be reasonably encountered in primary care settings. Additionally, specific populations of interest may be specified a priori by the working group, if a suitable rationale is provided (e.g., considerable variation in baseline rates or effects of the intervention appears to occur).
A choice on how broadly to define the population must be determined by underlying biological or other considerations. In other words, is it plausible that across the range of patients being considered, that the magnitude of effect of the intervention on key outcomes is more or less the same, or is it different?
3.5.2 Defining the Intervention
In relation to defining the intervention, Task Force recommendations address primary or secondary preventive interventions delivered in primary care settings or which are referable within primary care. Primary prevention interventions are those provided to individuals in a clinical setting to prevent the onset of a condition or disease while secondary preventive measures identify asymptomatic individuals in whom the condition has not become apparent (8). Screening is part of the clinical pathway to identify conditions and improve downstream health outcomes. Interventions that are part of the treatment and management of patients with a clinical condition or disease are outside the scope of the Task Force, however, in some cases their effects may be considered if used as indirect evidence for the potential impacts of screening.
The difference between screening and case-finding should be considered when recommendations for screening are being developed. Some groups use the terms “screening” and “case-finding” interchangeably. However, the Task Force maintains the following distinctions: screening is the examination of a population not known to have symptoms or suspected of disease, using a specific tool, to identify a condition of interest, whereas case-finding is the examination of an individual or group suspected of having the condition based on signs or symptoms, for instance.
The issue of timing, frequency and type of intervention can be addressed in the PICOTS as part of the definition of the intervention. The location, context, personnel, equipment and whether or not the intervention can be done alone or in combination with other interventions should also be identified.
3.5.3 Determining Comparators
The scoping exercise also assists members of the working group to determine the comparator(s) which should be included in the PICOTS. Clarity in choice of the comparator(s)–which could be a combination of comparators–ultimately makes the guideline more useful in primary care practice. The comparator(s) should be defined in as much detail as possible.
The intervention could be compared with, for example:
- Usual care, placebo, or no intervention;
- Another type of intervention;
- A combination of interventions; or
- Another frequency, duration, intensity, delivery method, or threshold for positivity of the same intervention.
3.5.4 Selecting and Rating Outcomes
As part of developing the PICOTS, the members of the working group develop a draft list of patient-important outcomes (i.e., benefits and harms), including any surrogate outcomes, that may result from the screening or prevention intervention. The guideline working group labels each outcome as benefit, harm or potential benefit/harm (for those which hypothesized directionality is less clear) to ensure they have a comprehensive set of outcomes and have not overlooked harms. The list is developed using information from the scoping exercise (e.g., natural history of disease, other reviews) and input from the working group and supporting groups (e.g., clinical experts). Examples of benefits resulting from the intervention in comparison to a control may include increased longevity, quality of life and reduction in morbidity, while examples of harms might include decreased longevity, surgical complications, psychological distress and other adverse effects.
Working group members and individuals supporting the working group are asked to independently rate outcomes. It is important to note that the selection of outcomes should be based on which are important for decision-making rather than those for which evidence is available. Ultimately, if evidence is not available for an outcome rated as critical or important, this should be acknowledged rather than ignoring the outcome (5,6).
Outcomes are rated from 1 to 9 in terms of their importance to decision-making on recommendations; with higher values being more important. Outcomes rated from 7 to 9 are considered critical for decision-making, those rated from 4 to 6 are important but not critical, and those rated from 1 to 3 are of limited importance and are not considered further (5). Working group members will consider the results of the outcome rating exercise and ultimately come to consensus on which outcomes will be included in the summary of findings and evidence profile tables.
The working group aims for a maximum of seven outcomes considered critical (rating 7–9) or important (rating 4–6) to be included in the summary of findings (9). The somewhat arbitrary number of seven outcomes has been proposed as reflective of the amount of information users can readily grasp (9,10). Outcomes considered critical (rating 7–9) are the primary factors influencing a recommendation; these are always used to determine the overall certainty of evidence supporting the recommendation (5). While important outcomes are included in the summary of findings tables, they may not influence the overall certainty of the evidence particularly if they are of low certainty while evidence available for critical outcomes is of higher certainty (5,8).
Factors to consider when rating outcomes:
- When developing guidelines for clinicians to care for patients, outcomes should be rated in relation to the patient’s perspective.
- The Task Force attempts to focus on outcomes it believes will be important for clinicians to discuss or highlight with a patient when presenting potential benefits and harms of a screening or preventive intervention.
- Judgements are relative, not absolute; this means that the Task Force weighs the importance of each outcome in relation to other relevant outcomes for the specific decision that is being considered.
- Judgements about ratings should not be swayed by whether working group members believe that there is no evidence for an outcome; rather surrogates for important outcomes should be identified when required.
3.5.4.1 Patient Values and Preferences
Involving patients/public in the process of developing health care guidelines is an important part of patient-centered care. When patients/public are involved in guideline development, developers can create guidelines that more accurately reflect patient preferences.
Patient/public input in the selection of outcomes can be obtained in different ways depending on the topic, including:
- Consult with a group of patients/public about proposed critical and important outcomes
- Survey a group of patients/public and ask them to rate the outcomes
- Review the literature for the importance patients place on outcomes (including studies of surveys, focus groups, interviews, utility ratings, other reviews, etc.). This review may be restricted to recent literature (e.g. <10 years ago) given the evolving nature of values and preferences.
3.5.4.2 Surrogate Outcomes
In general, the Task Force prefers to use clinically-relevant patient-important outcomes but will consider the use of surrogate outcomes if evidence is lacking (3).
There must be a relationship between the surrogate and the patient-important outcome, defined as the following:
- A high proportion of people with the surrogate outcome are expected to experience the condition or the outcome.
- An intervention directed toward the surrogate outcome leads to improvement or decline in the clinically relevant outcome.
Surrogate outcomes are important only to the extent that they reliably indicate changes in patient-important outcomes, where clinically relevant outcomes have not been measured and reported. The necessity to use a surrogate may lead to downgrading the certainty of the evidence in the final evidence-to-decision tables due to indirectness of the outcome measure (3,11). In this situation, the patient-important outcomes are specified with their associated surrogates in the analytic framework and summary of findings tables.
3.5.4.3 Non-patient-important Outcomes
In some cases, the Task Force may consider including non-patient-important outcomes (e.g., resource use or health system outcomes). Such outcomes may not be used in the weighing of benefits and harms, but are still important for considerations such as feasibility, acceptability, equity, resource use and implementation.
3.5.4.4 Mortality as an Outcome
Mortality is considered a critical outcome for most questions, but when it is not likely to be a direct consequence of a condition, or intervention, or a measurable outcome, it may not be included in the review. When mortality is a critical outcome, the Task Force may consider both all-cause mortality and cause-specific mortality in developing its recommendations.
In situations where the condition of interest commonly causes death, the Task Force may consider all-cause mortality, rather than cause-specific mortality, as a final health outcome. Any difference in the effect of the intervention between all-cause mortality and cause-specific mortality should also be considered. Such differences may be attributed to there being a benefit of the intervention for the condition of interest but an increase in mortality related to other conditions. Alternatively, the difference may occur because there is a decrease in cause-specific mortality but no change in all-cause mortality, which may indicate a potential harm of the intervention for patients with other conditions. Differences between all-cause and cause-specific mortality may also occur if the condition of interest is rare or if the population is subject to other causes of mortality, in which case the intervention has little or no effect on all-cause mortality (3).
Methodological issues may contribute to differences between all-cause and cause-specific mortality. Accurately ascertaining the cause of death for participants in clinical studies is potentially difficult, and deaths may be attributed to a condition even in cases where the condition did not contribute to the death. Conversely, when physicians know that their patients are involved in a study (as is the case for some interventions where blinding is impossible) and are uncertain of the actual cause of death, they may be reluctant to attribute the death to the condition of interest. This could lead to a false or inflated reduction in cause-specific mortality. Moreover, participants enrolled in the active-intervention arm of a trial may be followed more closely before death than those in the passive, no-intervention arm, which may mean that selected information about those in the intervention arm is available even to the external adjudicators of cause of death. This may in turn lead to biased estimates of cause-specific mortality.
3.5.5 Selecting Study Designs
The approach for determining the study designs that will be included to address KQs should be documented a priori and explained in the final protocol, to ensure that the process is transparent, defensible, and reproducible.
Once the KQs have been developed, the working group determines, with input from the ERSC, which study designs would be most appropriate. For the main benefit outcomes, members of the working group may determine whether they will focus exclusively on RCTs or whether they will expand the search to include non-randomized studies (12) and/or modelling studies (3,13). The working group should come to a consensus, based on a clear rationale, about the study designs that will be admissible for the review and should document these decisions. For harms, in an examination of less common/rare harms of an intervention, or long-term harms, the working group may decide to include non-randomized studies (e.g., large, cohort studies with longer follow-up periods), as evidence on harms may not be available from RCT evidence. Likewise, some harms such as false positive results will not be experienced by a control group and thus uncontrolled studies may be used if, for example, the rates of false positives in RCTs may be different than in large real-world studies that focus on efficacy.
The working group may also decide on a stepwise approach to the search itself, whereby data is first collected from RCTs and it is determined whether information from non-randomized studies or modelling data are needed once the RCT data have been reviewed. In such cases, the process and criteria for supplementing the RCT data should be documented in advance.
Some KQs, if not focusing on causation to an intervention, may not be best answered by RCTs. For example, questions on diagnostic accuracy, prognosis, patient preferences or acceptance are best answered by other study designs. Certain study design features may be implemented for these studies (e.g., excluding case-control studies on accuracy or development cohorts of risk prediction models), on a case-by-case basis.
3.5.6 Analytic Framework for Intervention Key Questions
The Task Force uses analytic frameworks to graphically represent KQs about complex interventions (14). The analytic framework defines and links clinical concepts, interventions and populations as they relate to outcomes. Each of the clinically relevant KQs is identified in the analytic framework (14,15). Patient preferences and resource use are not included in the framework, which focuses on the direct clinical pathway, but may be identified in the KQ list. The analytic framework is developed a priori and links the population, interventions, comparisons and outcomes to help structure the evidence review and ensure it provides the information required for the working group to make a recommendation(s) about the intervention (15).
It is important to note that analytic frameworks do not necessarily incorporate all factors associated with the screening or prevention intervention. Furthermore, they are not decision algorithms and do not incorporate all possible outcomes of an intervention – just those that are identified by the working group as being required to answer the KQs.
In an analytic framework, actions (such as the performance of a screening test) are depicted by arrows, and health outcomes are depicted by rectangles while KQs are numbered in a circle. It is not necessary to identify each outcome in the framework; where many outcomes are being considered, the phrase ‘patient-important outcomes’ could be used in the framework itself with definitions below; these outcomes are further divided into benefits and harms (14,15).
An analytic framework distinguishes between clinically relevant patient-important health outcomes and surrogate outcomes. A health outcome refers to a measure of health status while a surrogate is an outcome that can act as a proxy for the health outcome of interest. The association of surrogate outcomes to the final outcome is depicted with a dashed, rather than a solid, line. The analytic framework specifies the different components of the clinical path of the disease or disorder from beginning to end.
The analytic framework specifies populations, actions, and outcomes:
- The population consists of the individuals for whom the proposed screening or preventive service is intended.
- The actions link the population to the outcomes (or they may link outcomes directly) and may include screening and treatment. The name of each action appears in a label above its respective arrow.
- Clinically relevant health, or surrogate outcomes result from actions or from previous outcomes. These outcomes may be further described as benefits or harms to reflect anticipated effects.
Each arrow is associated with a KQ that will be addressed by the evidence review, and all of the KQs are listed within the analytic framework.
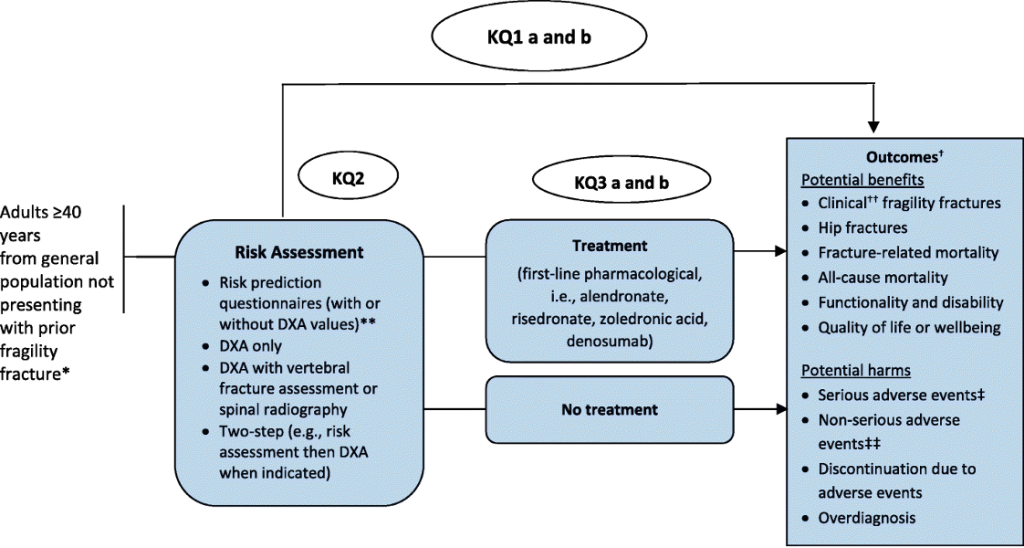
KQ1a: What are the benefits and harms of screening compared with no screening to prevent fragility fractures and related morbidity and mortality in primary care for adults ≥ 40 years?
KQ1b: Does the effectiveness of screening to prevent fragility fractures vary by screening program type (i.e., 1 step vs 2 step) or risk assessment tool?
KQ2: How accurate are screening tests at predicting fractures among adults ≥ 40 years?
KQ3a: What are the benefits of pharmacologic treatments to prevent fragility fractures among adults ≥ 40 years?
KQ3b: What are the harms of pharmacologic treatments to prevent fragility fractures among adults ≥ 40 years?
KQ4: For patients ≥ 40 years, what is the acceptability* of screening and/or initiating treatment to prevent fragility fractures when considering the possible benefits and harms from screening and/or treatment
Figure 2. Example of an analytic framework
3.6 Non-intervention Key Questions
Apart from the main KQ on the benefits and harms of screening or another preventive intervention, additional KQs may cover considerations such as:
- Appropriate intervals for screening
- Considerations for specific populations
- Patient values and preferences (e.g., relative importance of outcomes and/or preferences for different intervention attributes)
- Diagnostic test accuracy or risk prediction
- Prognosis
- Acceptability of screening by patients or providers
- Cost-effectiveness
Methods for developing these such KQs differ from those for intervention KQs and may not follow the PICOTS approach specified in section 3.5. The Task Force follows suitable current guidance for developing non-intervention KQs (guidance followed will depend on question type). Additional considerations for certain types of non-intervention KQs are provided in the sections below.
3.6.1 Patient Values and Preferences
The Task Force strives to focus on patient-important outcomes when reviewing the evidence on the effectiveness of interventions and for making their recommendations. One or more KQs may be included when there is a need for a more comprehensive assessment of patient perspectives to inform guideline development. The requirement for a KQ on patient values and preferences is dependent on the outcomes identified for the intervention supporting a staged approach to the evidence review as described in the following scenarios:
| Evidence on Intervention | Patient Values and Preferences (PVP) Requirement |
| Evidence of overall desirable outcomes | Systematic Review (SR)* on PVP should be conducted |
| Evidence of overall undesirable outcomes and/or intervention clearly harmful | SR on PVP does not need to be conducted |
| Intervention probably harmful | SR* on PVP should be conducted |
| Unclear balance of desirable and undesirable outcomes | SR* on PVP should be conducted |
*In some situations, a SR may not be feasible given resource or other constraints.
3.6.2 Consideration of Resource Use
The Task Force is mindful that screening and preventive interventions consume scarce primary care resources, such as available provider time (16,17). Where resource use is deemed to be critical or important for making decisions on recommendations, questions addressing resource use related to implementing an intervention may be included in the evidence review as a KQ(s). There may also be consideration of performing a de novo cost-effectiveness analysis or using one that aligns well with the scope of the review, if feasible. Questions on resource use may be part of a ‘staged’ process and answered only if the intervention is determined to have a net beneficial impact on patient-important outcomes.
The focus from a GRADE perspective is on identifying and rating confidence in evidence on differences in resource use between options being compared. Ultimately, an intervention with even a small beneficial impact may be recommended. For example, an intervention with very low costs may be recommended, while an intervention which uses a significant amount of resources may not be recommended even if it has a greater impact (3,18).
When resources are considered, the Task Force usually takes the perspective of the health care payer or the societal perspective when making recommendations (3,18). This means that changes in the use of health care resources which result from the intervention (i.e., health care payer perspective) are considered as well as non-health care resources including patient and informal caregiver resources and changes in productivity (i.e., societal perspective). Assessment of these costs supports an economic evaluation of the intervention such as an analysis of cost-effectiveness, cost-utility and cost-benefit (18).
3.7 Final Scoping Document
The final scoping document includes background information on the topic, list of key systematic reviews and randomized controlled trials, comparative analysis of relevant guidance and identification of gaps. Certain sections will also help serve the working group for guideline considerations (e.g., burden of disease, if treatment effects are summarized in detail using high quality evidence and this is used rather than a KQ as part of rationale for screening or other parts of recommendation). Appendix outlines the components of the scoping document. Not all of the items may be relevant to every topic and they may need to be adapted accordingly and in response to the working group needs.
3.8 Feasibility Assessments and Final Feasibility Report
The ERSC develops feasibility assessments based on the decisions made by the working group during the scoping process (e.g., the desired KQs and PICOTS elements). Feasibility assessments include expected search yields, budget and timelines for different approaches to KQ searches (e.g., different year limitations). Scoping and feasibility assessments are often carried out in parallel (e.g., the ERSC may examine expected search yields of different PICOTS options being considered by the working group to inform their decision) and can become iterative processes (e.g., adjusting scope, PICOTS, additional searches and systematic reviews based on anticipated feasibility), especially when dealing with large bodies of evidence. The scope of KQs, particularly those that may not be critical (e.g., comparative accuracy) may be modified due to resource constraints. The final feasibility report details the search strategy, budget and timelines for each KQ to be included in the evidence review protocol. The Working Group Chair, Science Team Lead and Science Team Manager review and approve this report before progressing to the protocol stage of the guideline development process.
References
- Alonso-Coello P, Schünemann HJ, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ. 2016 Jun;353:i2016.
- Alonso-Coello P, Oxman AD, Moberg J, Brignardello-Petersen R, Akl EA, Davoli M, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 2: Clinical practice guidelines. BMJ. 2016 Jun;353:i2089.
- Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE handbook for grading the quality of evidence and strength of recommendations [Internet]. 2013 [cited 2020 Jan 30]. Available from: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html
- Samson D, Schoelles KM. Chapter 1: Introduction to the Methods Guide for Medical Test Reviews [Internet]. Vol. 27, Journal of General Internal Medicine. 2012 [cited 2022 Nov 28]. Available from: https://effectivehealthcare.ahrq.gov/products/methods-guidance-tests-introduction/methods
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011 Apr;64(4):395–400.
- O’Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014 Jan;67(1):56–64.
- The Cochrane Collaboration. PROGRESS-Plus [Internet]. 2022 [cited 2022 Mar 31]. Available from: https://methods.cochrane.org/equity/projects/evidence-equity/progress-plus
- Last J. A dictionary of public health. Oxford: Oxford University Press; 2007.
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston R V, Thomas J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6 [Internet]. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). 2021 [cited 2021 Oct 15]. Available from: www.training.cochrane.org/handbook
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013 Feb;66(2):158–72.
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011 Dec;64(12):1303–10.
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol. 2019 Jul;111:105–14.
- Woolf S, Schünemann HJ, Eccles MP, Grimshaw JM, Shekelle P. Developing clinical practice guidelines: types of evidence and outcomes; values and economics, synthesis, grading, and presentation and deriving recommendations. Implement Sci. 2012 Jul;7:61.
- Agency for Health Care Research and Quality. Analytic Frameworks [Internet]. [cited 2018 May 10]. Available from: https://www.slideshare.net/AHRQEHCProgram/analytic-frameworks
- Guise J-M, Chang C, Butler M, Viswanathan M, Tugwell P. AHRQ series on complex intervention systematic reviews-paper 1: an introduction to a series of articles that provide guidance and tools for reviews of complex interventions. J Clin Epidemiol. 2017 Oct;90:6–10.
- Thombs BD, Straus SE, Moore AE. Update on task force terminology and outreach activities: Advancing guideline usability for the Canadian primary care context. Can Fam Physician. 2019 Jan;65(1):12–3.
- Privett N, Guerrier S. Estimation of the Time Needed to Deliver the 2020 USPSTF Preventive Care Recommendations in Primary Care. Am J Public Health. 2021 Jan;111(1):145–9.
- Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013 Feb;66(2):140–50.