Hepatitis C—Clinician FAQ
We recommend against screening for hepatitis C virus (HCV) in adults who are not at elevated risk. Strong recommendation
1. Why is the recommendation against screening?
- Given the lack of direct evidence on the benefits and harms of screening and the very low quality of the indirect evidence on long-term benefits, substantial uncertainty remains about the effectiveness of screening adults not at elevated risk in Canada.
- Treatment for HCV also raises many issues that led to this recommendation:
- There is uncertainty about the true long-term benefit of treating individuals detected through screening, many of whom would never develop clinical disease even without treatment.
- It is extremely expensive to treat HCV and at current prices, it is not possible for drug plans to fund treatment for all asymptomatic HCV-positive individuals.
- Even if markedly lower drug prices were available, changes to models of care may also be required before population-based screening could be warranted, such as changes in health system policies to support a successful roll out of a treatment strategy that would include all individuals identified with chronic HCV infection, regardless of fibrosis stage or comorbidity.
- Current eligibility criteria for treatment include reaching later stages of liver disease (based on degree of hepatic fibrosis), so individuals may be identified as having HCV through screening but will be unable to receive treatment if they are asymptomatic or in the early stages of liver disease.
- Potential to increase inequity as only some people would be able to afford the high cost of paying for treatment out of pocket.
- Better access to direct-acting antiviral-based treatment may require extending management of HCV to clinicians in primary care.
2. Who does this recommendation apply to?
- This recommendation applies only to adults who are not at elevated risk for HCV.
- It does not apply to pregnant women.
3. Why is this recommendation strong?
- The CTFPHC is confident about the potential for harm resulting from screening (e.g., overdiagnosis, anxiety, and stigma) and treatment (e.g., side effects from medication) for HCV.
- The cost of treatment is high.
- There is also considerable doubt about the benefits of screening.
4. Is your patient at elevated risk of hepatitis C? It is important to talk with them about testing if they belong to any of the following categories:
- Current or past history of injection drug use
- Have been incarcerated
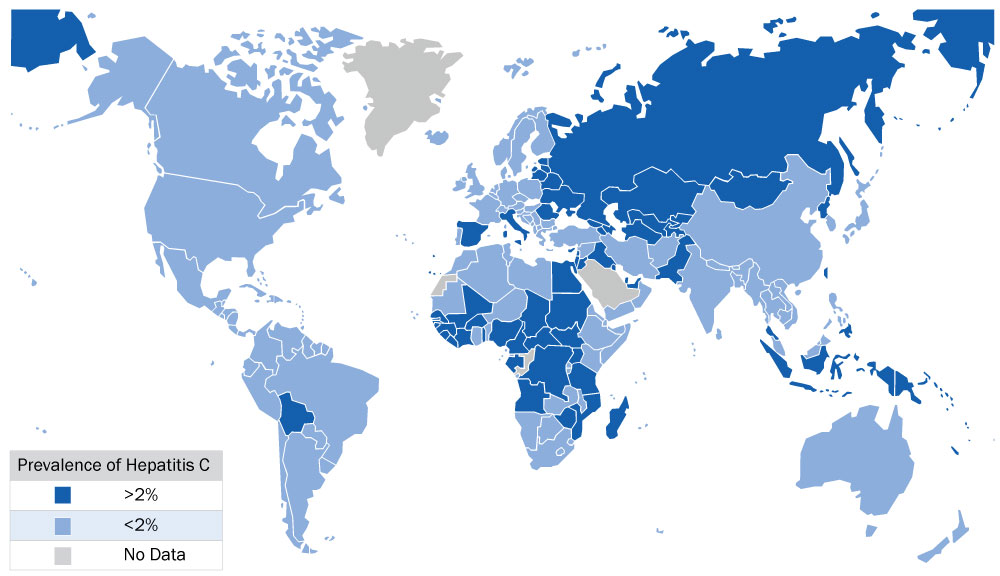
- Born, travelled or resided in HCV-endemic countries (see opposite for map)
- Received health care where there is a lack of universal precautions
- Recipients of blood transfusions, blood products, or an organ transplant before 1992
- Hemodialysis patients
- Individuals who have had needle stick injuries
- Other risks sometimes associated with HCV exposure, such as:
- High-risk sexual behaviours, homelessness, intranasal and inhalation drug use, tattooing, body piercing, or sharing sharp instruments or personal hygiene materials with someone who is HCV positive.
- Anyone with clinical clues suspicious for HCV infection (and above risk factors).
It is important to note that risk within these categories varies. Some groups are at very high risk (e.g., injection drug users) and others have a lower risk (e.g., those who have travelled to HCV endemic countries). For more information on groups at elevated risk, please see CFPC/PHAC’s resource on Primary Care Management of Chronic Hepatitis C: http://www.cfpc.ca/uploadedFiles/Resources/Resource_Items/HEP_C_Guide_eng_2.pdf
Prevalence of Hepatitis C Virus Worldwide
| List of intermediate and high HCV-endemic countries by region1 | |
| East Asia & Pacific | American Samoa, Cambodia, China, Fiji, Indonesia, Japan, Kiribati, Fed. Sts. Micronesia, Mongolia, Palau, Papua New Guinea, Philippines, Solomon Islands, Taiwan, Tonga, Vanuatu |
| East Europe & Central Asia | Armenia, Azerbaijan, Belarus, Estonia, Georgia, Hungary, Kazakhstan, Kyrgyz Republic, Latvia, Lithuania, Macedonia FYR, Moldova, Poland, Romania, Russian Federation, Tajikistan, Turkey, Turkmenistan, Ukraine, Uzbekistan |
| Latin America & Caribbean | Bolivia, El Salvador, Grenada, Haiti, St. Kitts and Nevis |
| Middle East & North Africa | Egypt, Iraq, Jordan |
| Sub-Saharan Africa | Angola, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, De. Rep. Congo, Côte D’Ivoire, Gabon, Gambia, Guinea, Guinea-Bissau, Liberia, Malawi, Mali, Mauritius, Mozambique, Niger, Nigeria, Rwanda, São Tomé and Principe, Senegal, Sierra Leone, Sudan, Tanzania, Togo, Uganda, Zimbabwe |
See Appendix 6 in Recommendations on Hepatitis C Screening for Adults by the Canadian Task Force on Preventive Health Care for the prevalence percentage for each country.
1 Greenaway C, Ma AT, Kloda LA, Klein M, Cnossen S, Schwarzer G, Shrier I. The Seroprevalence of Hepatitis C Antibodies in Immigrants and Refugees from Intermediate and High Endemic Countries: A Systematic Review and Meta-Analysis, S4 Appendix. Country specific estimate. PLoS ONE 2015;November 11:doi:10.1371/journal.pone.0141715.s004.